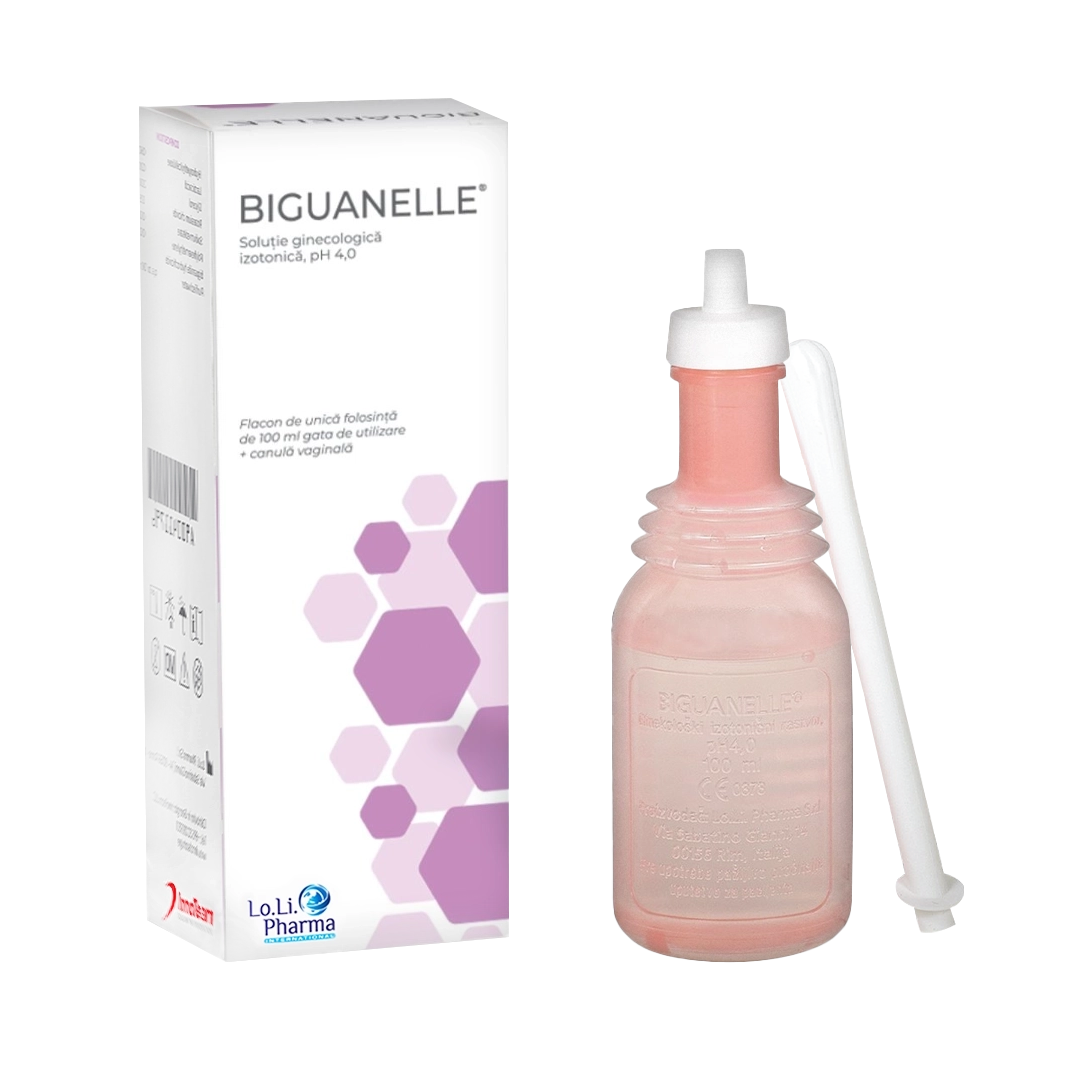
Biguanelle
Active Substance: hydroxyethyl cellulose (0.60 g), lactic acid (0.20 g), glycerol (2.00 g), potassium chloride (O.15 g), sodium edetate (0.10 g), polyhexamethylene biguanide hydrochloride (PHMB) (0.10 g), purified water (q.s.to 100 ml).
Pharmacology Group: Single-use Medical Device
Manufacturer: LO.LI. Pharma International
Country: Italy
Package Form: One 100 ml bottle with vaginal cannula
Delivey Form: III group, non-prescription
Biguanelle
Isotonic Gynaecological Solution, pH 4.0
PROPERTIES
The use of BIGUANELLE helps restore natural physiological functions, even during traumatic pharmacological or physical treatments. The use of BIGUANELLE during the treatment of vaginal disorders or after physical treatment helps restore normal vaginal physiology. The product is preserved with polyhexanide (PHMB), a cationic surfactant well tolerated by all mucosa.
INDICATIONS
Treatment of vulvovaginitis and cervicovaginitis of any origin and nature by restoring and maintaining the natural physiology of the vaginal environment. Adjuvant in re-epithelialization processes following physical treatment (diathermocoagulation, laser therapy, cryotherapy), exerting a protective, barrier effect in adult women.
CONTRA-INDICATIONS
Known hypersensitivity to the components.
SIDE-EFFECTS
In some cases, intolerance (burning or irritation) may occur. However, this does not have any consequences and does not require any changes to the treatment. Tell your doctor or pharmacist about any undesirable effects you suspect may have been caused by using the product.
INCOMPATIBILITIES / INTERACTIONS
To date, there are no known interactions with other devices, substances or medicinal products.
EFFECTS ON THE ABILITY TO DRIVE AND USE MACHINES
No effects on the ability to drive and use machines have been reported.
DOSAGE AND METHOD OF ADMINISTRATION
One single application is sufficient and can be repeated, if necessary, after 4 days as advised by a doctor.
INSTRUCTIONS FOR USE
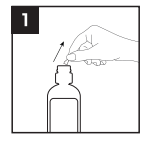
1. Remove the sealed cap by pushing it to the side;
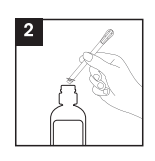
2. Remove the cannula from the protective packaging and insert it in the bottle, making sure it is securely positioned:
3. Choose a suitable position, which will allow the solution to stay inside the vagina when taking effect;
4. Carefully insert half the cannula into the vaginal canal;
5. Squeeze the bottle, until it is completely empty. Allow the liquid to remain in the vagina for a few minutes so the solution can take effect. You should also follow any instructions given by your treating doctor.
WARNINGS
Use only once and discard the single-dose container with any residual content. The use of any product for topical use, especially if prolonged, can lead to sensitisation; speak to your doctor if this occurs. The product should be administered vaginally only. No injuries from overdose have been reported under normal conditions of use. Use during pregnancy must be evaluated by medical consultation. Do not use the product, if you are allergic to any of the components, after the expiry date or if the box is not intact. Keep out of reach of children. Do not swallow. Any serious incident, that has occurred in relation to the medical device, should be reported to the manufacturer and the competent authority of the Member State in, which you are established.
PRECAUTIONS FOR STORAGE
Store at room temperature (≤ 25 °C), protected from light and in a cool place.
PACKAGE
One 100 ml bottle with vaginal cannula.
Manufacturer: LO.LI. Pharma International. Italy
Related Products
INOFOLIC HP
Active Substance: Myo-inositol (2000 mg), alpha-lactalbumin (50 mcg) and folic acid (200 mcg). Pharmacology Group: Dietary supplement Manufacturer: LO.LI. Pharma International Country: Italy Package Form:…
Biosteron
Active Substance: dehydroepiandrosterone Pharmacology Group: Glucocorticoids → Glucocorticoid means for oral use Manufacturer: Lek-am Country: Poland Package Form: 25 mg tablet #60 Delivey Form: Group…
Hyalo Gyn vaginal gel
Active Substance: Hydeal-D 0.2% (hyaluronic acid derivate) Pharmacology Group: Medical device. Obstetrics, Gynaecology → regulates vaginal microflora→ Drugs, that activate metabolism in tissues, improve trophism…
Andrositol Plus
Active Substance: Myo-inositol (1000 mg), N-acetylcysteine (600 mg), Vitamin E (30 mg), L-carnitine (30 mg), L-arginine (30 mg), alpha-lactalbumin (25 mg), folic acid (200 mcg)…